Trulance
What is Trulance (Plecanatide)?
Living with chronic constipation can be more than just uncomfortable, it can disrupt daily life, affect mood, and make even routine tasks difficult. Trulance (plecanatide) offers a scientifically backed yet patient-friendly option for adults struggling with persistent constipation. As a prescription medication designed to improve bowel regularity and comfort, Trulance helps restore a sense of normalcy and well-being for those whose digestive symptoms haven’t improved with diet or over-the-counter remedies.
Approved by the U.S. Food and Drug Administration (FDA) in 2017, Trulance belongs to a class of drugs known as guanylate cyclase-C agonists. It works locally in the intestines, meaning its effects are confined to the digestive tract and is used as a first-line or adjunct therapy for specific forms of chronic constipation in adults.
What does Trulance do?
Trulance is prescribed to treat two main conditions:
- Chronic idiopathic constipation (CIC): ongoing constipation without a known medical cause.
- Irritable bowel syndrome with constipation (IBS-C): a subtype of IBS where patients experience abdominal discomfort, bloating, and infrequent bowel movements.
By helping the intestines retain more water and improving stool consistency, Trulance promotes more natural and complete bowel movements. Unlike some stimulant laxatives that can cause urgency or cramping, Trulance supports a gentler, more physiologic pattern of bowel function.
Clinical studies have shown that many patients taking Trulance experienced increased stool frequency and improved stool texture within the first week of therapy, along with meaningful reductions in abdominal discomfort and straining (FDA, 2017; Mayo Clinic, 2024).
How does Trulance work?
Trulance works by activating guanylate cyclase-C receptors on the inner surface of the small intestine. When these receptors are stimulated, they increase levels of cyclic guanosine monophosphate (cGMP), a molecule that helps regulate fluid balance and bowel motility.
Here’s how this mechanism helps patients:
- It increases fluid secretion into the intestines, softening stool and making it easier to pass.
- It stimulates natural intestinal muscle contractions, encouraging more regular bowel movements.
- It may also reduce visceral pain signals, which helps ease abdominal discomfort commonly experienced in IBS-C.
Because Trulance acts locally within the gastrointestinal tract and is minimally absorbed into the bloodstream, it carries a low risk of systemic side effects, a key advantage for long-term therapy.
Trulance side effects
Most people tolerate Trulance well, but as with any prescription drug, some may experience side effects.
Common side effects include:
- Diarrhea (the most frequently reported)
- Bloating or gas
- Abdominal discomfort
Less common side effects:
- Dizziness
- Fatigue
- Nausea
Serious side effects are rare, but patients should contact a healthcare provider immediately if they experience:
- Severe or persistent diarrhea leading to dehydration
- Signs of an allergic reaction such as rash, swelling, or difficulty breathing
Who should avoid Trulance: Trulance should not be used in children under 6 years of age due to the risk of severe dehydration. It’s also not recommended for individuals with known or suspected mechanical bowel obstruction (NIH, 2024).
Overall, Trulance is considered safe for adult use under medical supervision. Most side effects are mild and transient, resolving as the body adjusts to treatment.
Trulance dosage
Trulance comes as an oral tablet that is typically taken once daily, with or without food. The tablet should be swallowed whole, not crushed or chewed to ensure proper delivery to the intestines.
Your healthcare provider will determine the appropriate regimen based on your diagnosis (CIC or IBS-C) and individual response to treatment. While routine blood tests are generally not required, your doctor may monitor your hydration status or bowel pattern during the first few weeks to ensure the medication is well-tolerated.
Special considerations:
- Older adults and those with mild kidney or liver issues usually do not require dose adjustments.
- If diarrhea occurs, your doctor may recommend pausing the medication temporarily to avoid dehydration.
Does Trulance have a generic version?
As of 2025, no generic version of Trulance (plecanatide) is approved by the FDA. It is available only as the brand-name product marketed by Salix Pharmaceuticals. The company offers patient assistance and copay savings programs that can help make treatment more affordable. However, international versions may exist in other markets.
When a generic form becomes available in the future, it will be held to the same standards of quality, strength, and effectiveness as the brand-name version, ensuring equal clinical benefit at a lower cost (FDA, 2024).
Conclusion
Trulance represents an important advancement in managing chronic constipation and IBS-C. By acting directly on the intestinal surface to promote natural fluid balance and bowel regularity, it provides a well-tolerated, effective, and non-stimulant option for adults seeking lasting relief.
Patient experiences may vary, some notice improvements within days, while others may need a few weeks of consistent use. What remains constant is that Trulance, when prescribed and monitored by a qualified healthcare provider, is a safe and clinically proven therapy that supports digestive comfort and overall quality of life.
If you’re living with ongoing constipation, talk to your doctor about whether Trulance could be right for you. A personalized approach to digestive health can make all the difference in regaining daily confidence and comfort.
References
- U.S. Food and Drug Administration. (2017). Trulance (plecanatide) prescribing information. Retrieved from https://www.fda.gov
- Mayo Clinic. (2024). Plecanatide (oral route): Description and side effects. Retrieved from https://www.mayoclinic.org
- National Institutes of Health. (2024). Plecanatide – Drug information. Retrieved from https://medlineplus.gov
Approved To Treat
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
- TRULANCE is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile mice, administration of a single oral dose of plecanatide caused deaths due to dehydration
- Avoid use of TRULANCE in patients 6 years to less than 18 years of age
- The safety and effectiveness of TRULANCE have not been established in patients less than 18 years of age
- chronic idiopathic constipation (CIC).
- irritable bowel syndrome with constipation (IBS-C).
- Patients less than 6 years of age due to the risk of serious dehydration
- Patients with known or suspected mechanical gastrointestinal obstruction.
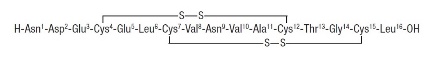
30 Tablets
(plecanatide) tablets
Dispense the accompanying
Medication Guide to each patient.


